Follow us on Telegram for the latest updates: https://t.me/mothershipsg
Those in Singapore can now get their doses of bivalent Moderna/Spikevax vaccine from as early as Oct. 14 onwards.
This is three days earlier than the previous date announced by the Ministry of Health (MOH), which was Oct. 17.
Infections rising
In a media doorstop on Oct. 11, health minister Ong Ye Kung revealed that their operations teams were able to complete their preparations for the rollout of the bivalent vaccine earlier, and are therefore administering it early.
There are also benefits to administering it early, as infections are rising due to the XBB Omicron subvariant.
The bivalent Moderna/Spikevax vaccine is based on the same original vaccine, with the same dosage for boosting.
However, instead of only targeting the original Covid-19 virus, this vaccine also targets the Omicron variant.
It will thus provide better protection against newer Covid-19 variants.
Both Ong and co-chair of the Multi-Ministry Taskforce Gan Kim Yong received their doses of the bivalent Moderna/Spikevax vaccine today (Oct. 11) at the Joint Testing & Vaccination Centre (JTVC) at Commonwealth.
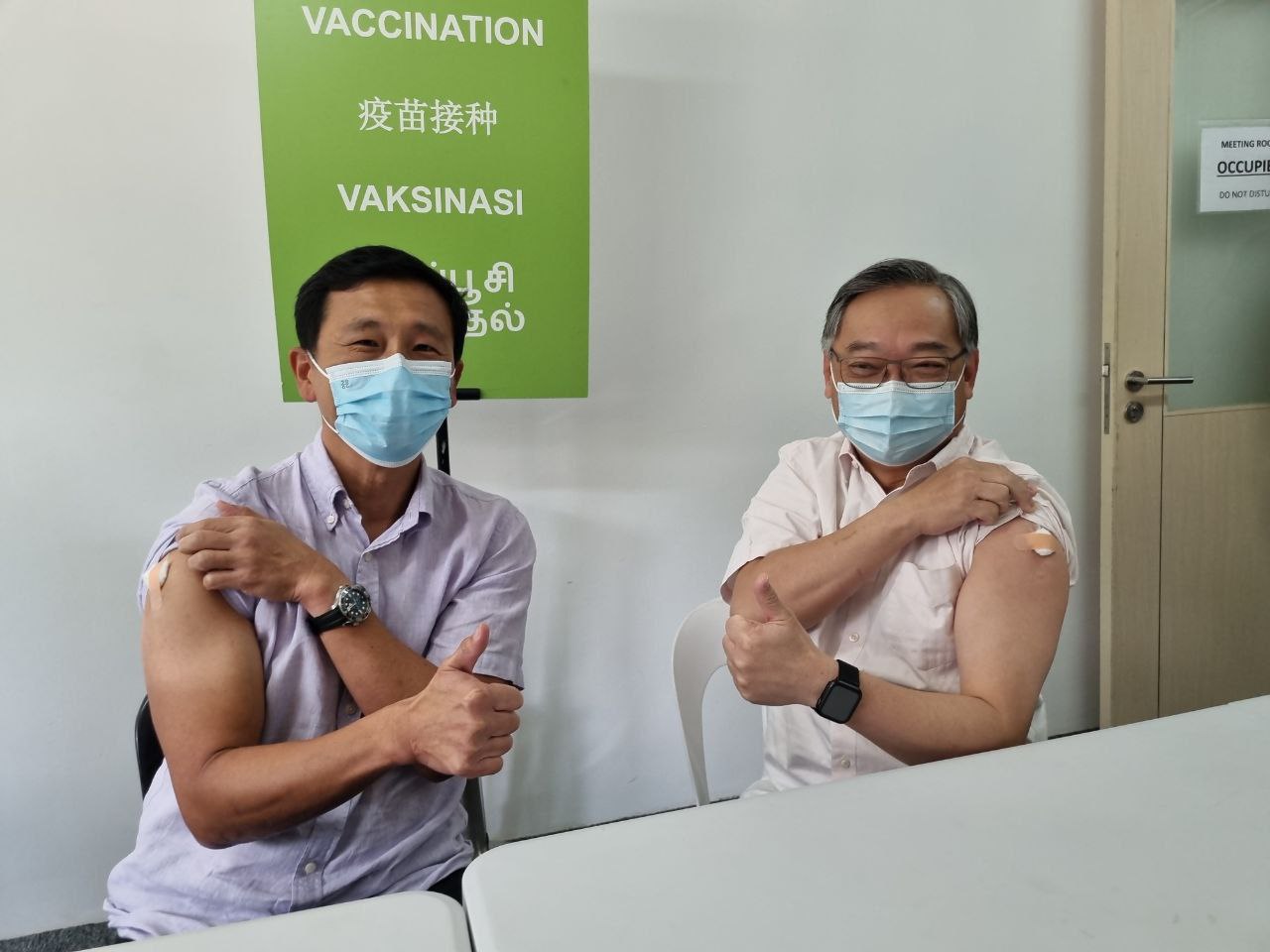 Photo by Isaac Wong
Photo by Isaac Wong
From Oct. 14, persons who have yet to achieve minimum protection, or those aged 50 and above who have received their last vaccine dose more than five months ago, may get the bivalent Moderna/Spikevax vaccine.
They can do so at any of the nine JTVCs offering the Moderna/Spikevax vaccines.
- JTVC Ang Mo Kio
- JTVC Bukit Merah
- JTVC Commonwealth
- JTVC Jurong East
- JTVC Kaki Bukit
- JTVC Pasir Ris
- JTVC Sengkang
- JTVC Woodlands
- JTVC Yishun
Individuals can also refer to this website to locate the nearest JTVC and their operating hours.
No safety concerns reported from bivalent Moderna/Spikevax vaccine
MOH explained that the approach and process of updating a vaccine with new strains of a disease is an established practice.
For example, influenza vaccines can include four strains of the influenza virus and can be updated twice a year.
According to post market surveillance, over 11 million updated bivalent mRNA vaccine doses have been administered in the U.S. to date without any safety concerns reported.
Modified and clinical studies have shown that the bivalent Moderna/Spikevax vaccine is "safe and efficacious."
A clinical trial for the vaccine in adults aged 18 and above compared 437 participants receiving the bivalent vaccine and 377 participants receiving the original booster vaccine.
The bivalent vaccine provided better protection against newer variants, generating an antibody response against the Omicron variant that was 75 per cent greater than the original booster.
The side effects after vaccination were similar for both vaccines and there were no safety issues observed.
The Health Sciences Authority and the Expert Committee on Covid-19 Vaccination have independently reviewed the date, and assessed that the bivalent Moderna/Spikevax vaccine has a similar safety profile as the original Moderna/Spikevax vaccine.
Risk of serious adverse events from the vaccine is very rare, MOH added.
Top photo by Isaac Wong
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.
