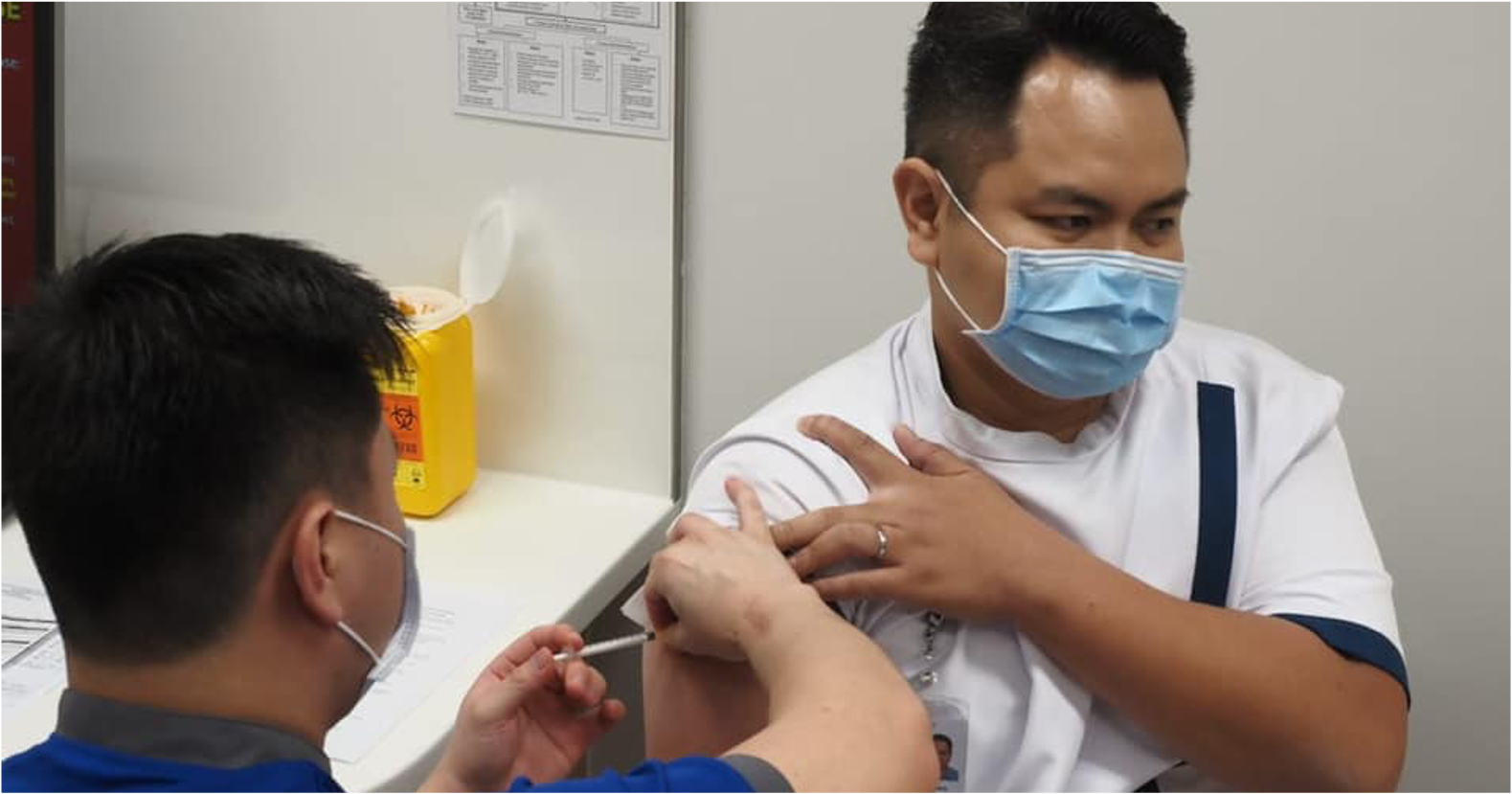
Singaporeans aged 40 to 44 will be able to register for Covid-19 vaccinations from May 19, 2021, according to a press release from the Ministry of Health (MOH)
The age-band will be extended, from the current range of those aged 45 and above, for both Pfizer and Moderna vaccines.
This was discussed during a technical briefing on May 18.
Former Health Minister Gan Kim Yong, currently the Trade and Industry Minister previously said in Parliament that those below 45 will be progressively extended invitations to get vaccinated from the latter half of May.
Vaccine eligibility age extended, 12 to 15
In addition, it was also announced that the age of those eligible to receive vaccines has been extended.
The Health Sciences Authority (HSA) has granted interim authorisation for the Pfizer-BioNTech vaccine to be administered to children from 12 to 15.
However, they will not be allowed to register for the vaccine yet, the window has not yet been opened for them at this point in time.
The Expert Committee on Covid-19 Vaccination has endorsed this recommendation, and also made the same recommendation to the Ministry of Health (MOH).
MOH will be working with the Ministry of Education to refine vaccination strategies for those aged 12 to 15, as almost all will be students.
HSA on vaccines for ages 12 to 15
The Pfizer-BioNTech vaccine was granted interim authorisation under the Pandemic Special Access Route (PSAR) for individuals aged 16 years and above in December 2020.
Data for individuals younger than 16 was not available then. As a condition for interim authorisation, Pfizer and BioNTech were required to continue to study the safety and efficacy of the vaccines in other subgroups, and submit the relevant data for HSA to review.
Pfizer and BioNTech submitted an application on April 13 to extend the vaccination population for the vaccine for adolescents aged 12 to 15 years old to HSA.
HSA’s review of the clinical data for this subgroup found that the vaccine induced a robust immune response and demonstrated a high vaccine efficacy of 100 per cent.
This vaccine efficacy was based on the on-going Phase 3 clinical trial, which enrolled over 2,000 participants aged 12 to 15 years.
Based on the safety data available from a median follow-up duration of two months after vaccination, the overall safety profile of the vaccine in adolescents was comparable to that observed in adults.
The side effects include injection site pain, fatigue, headache, chills and fever. They generally resolved on their own within a few days.
Pfizer and BioNTech will continue to follow up on the safety and efficacy of the vaccine in the clinical study for up to two years to determine its full safety profile in this subgroup.
HSA will also continue to closely monitor the safety of the vaccine to ensure that it continues to be safe for use in this population.
Related story:
Top image from MOH's Facebook page.
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.