UPDATE: This explainer was updated on December 14, following news that Singapore has approved the use of the Pfizer-BioNTech vaccine for pandemic use.
Singapore has approved the Pfizer-BioNTech vaccine for pandemic use.
More vaccines are expected to arrive in Singapore in the coming months
U.S. biotech firm Moderna is already in talks with Singapore's Health Sciences Authority (HSA) to bring its vaccine into the country.
There are many different Covid-19 vaccine candidates that are being developed right now. This website tracks those that are in Phase 1 to 3 of trial and some in research stages. All of them are racing against time to produce viable Covid-19 vaccines for the world.
But before we get ahead of ourselves, it is useful to understand how Covid-19 vaccines differ in the way they work.
How do Covid-19 vaccines trigger the body's immune response?
Vaccines generally work by triggering the body's immune response. The body then remembers it and activates the same response when it is infected.
However different vaccines trigger the body's immune response differently.
For the purpose of this piece, we will focus on four common types: Inactivated vaccines, Vector vaccines, Subunit vaccines, and mRNA vaccines.
Inactivated vaccines
Inactivated vaccines use a "killed" version of the virus, which is treated with UV light or chemicals so that it cannot cause disease.
The killed virus is then introduced into the body so that its antigens (the components which stimulate the immune system) will trigger an immune response.
This method is used in vaccines that combat polio and rabies.
Inactivated vaccines are typically not as strong as live vaccines, so several doses are needed over time.
This is why some vaccines are administered with an initial dose, followed by booster shots later on.
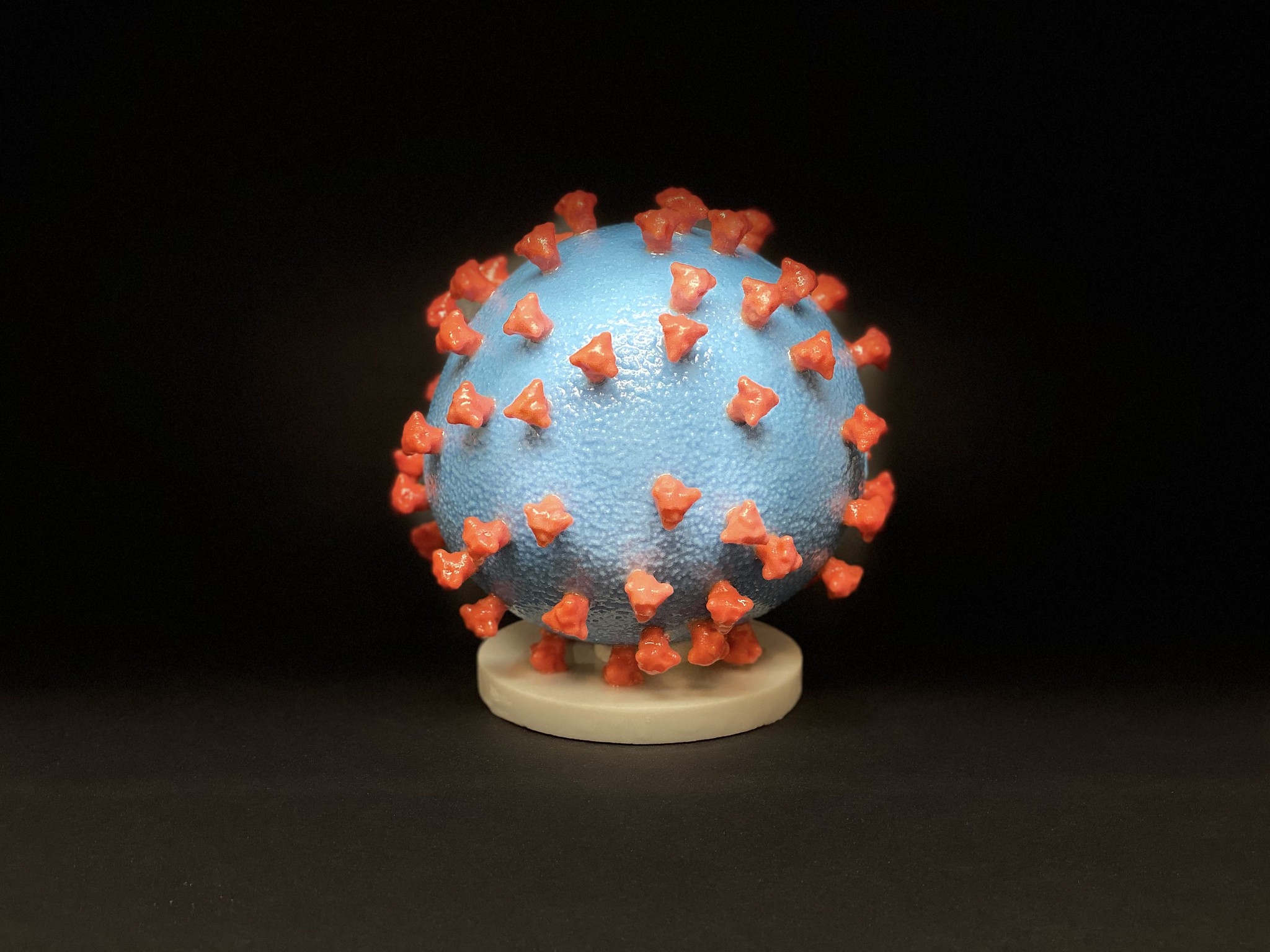 A 3D print of SARS-CoV-2. The entire virus — albeit not a live one — is used in inactivated vaccines. Image via.
A 3D print of SARS-CoV-2. The entire virus — albeit not a live one — is used in inactivated vaccines. Image via.
CoronaVac by Sinovac is an example of an inactivated vaccine.
One of CoronaVac's advantages is that it can be stored in a refrigerator at 2-8°C, without the need for for super-cool storage like those used for mRNA vaccines.
This means that CoronaVac is useful for countries which do not have the means to store vaccines at super-cool temperatures. For instance, Indonesia has already received 1.2 million doses of CoronaVac.
Vector vaccines
Vector vaccines work by using a weakened version of a live virus. The vaccine containing the weakened virus is called a viral vector.
In the case of Covid-19 vector vaccines, this weakened virus is not SARS-CoV-2 (the virus that causes Covid-19), but has genetic material that is similar to it.
The viral vector instructs our cells to make copies of a harmless protein — in the case of the vaccine for Covid-19, these are the "spike proteins" that are characteristic of the coronavirus.
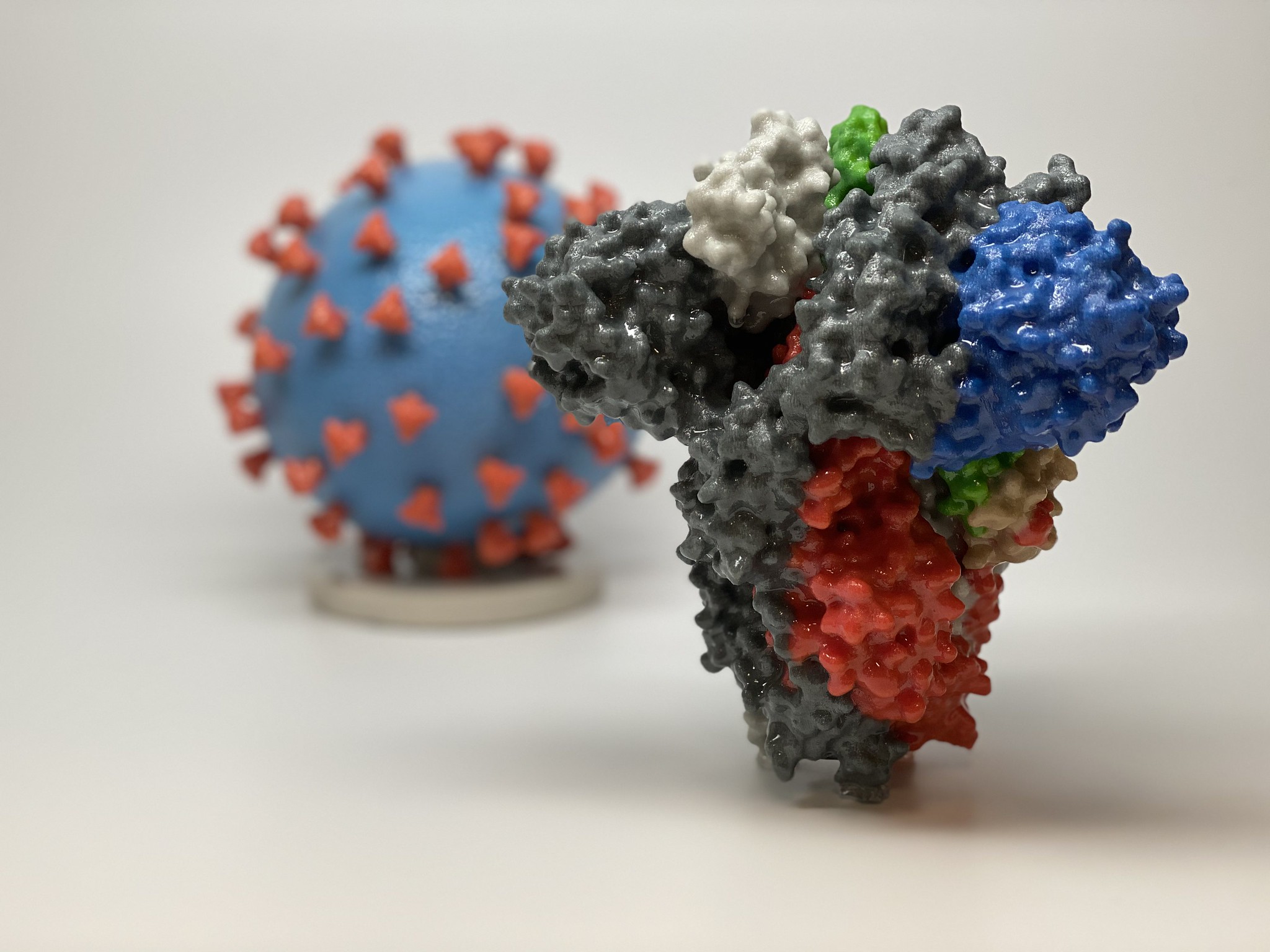 A 3D print of the spike protein found on the surface of SARS-CoV-2. Image via.
A 3D print of the spike protein found on the surface of SARS-CoV-2. Image via.
Since the virus is weakened, it will not spread and cause disease, but our immune system will recognise that it is foreign, and launch an attack.
If and when we get infected with SARS-CoV-2 in the future, our immune system will recognise it from its spike proteins, and mount the same response.
AZD1222 by AstraZeneca and JNJ-78436735 by Janssen are examples of vector vaccines.
They use weakened versions of the adenovirus — AZD1222 uses an adenovirus that causes infections in chimpanzees while Ad26.COV2.S uses a human adenovirus.
Subunit vaccines
Instead of the entire virus, subunit vaccines only include components of the virus that will stimulate the immune response. These components are called antigens.
Subunit vaccines typically have an additional component (called an adjuvant) to enhance the efficacy of the vaccine.
Once the subunit vaccine enters our bodies, our immune system recognises that the antigens are foreign entities. This triggers the immune system to attack this "foreign invader".
If we're infected by Covid-19 in future, our body will remember to recognise the antigens from the virus and mount the same attack.
NVX-CoV2373 from Novavax is a protein subunit vaccine.
Novavax first inserts the gene for the SARS-CoV-2 spike protein into moth cells which then produces the spikes on their membranes. These spike proteins are harvested and used in the vaccine, along with an adjuvant derived from trees.
mRNA vaccines
Covid-19 mRNA vaccines (or, messenger RNA vaccines) use material that is genetically coded to instruct our cells to produce the spike protein unique to SARS-CoV-2.
Once the vaccine is injected, our muscle cells produce the protein, triggering our immune system to launch an attack. In future, if we get infected with SARS-CoV-2, our body will remember how to fight that virus if we are infected in the future.
With an mRNA vaccine, the immune system is able to have a "preview" of what the Covid-19 virus would look like, without actually getting the disease.
One advantage of mRNA vaccines is that they can be produced in less time compared to the more traditional vector vaccines.
However, mRNA vaccines are notoriously unstable. They have to be kept at super-cool temperatures (Moderna's vaccine needs to be stored at -20°C and Pfizer's vaccine at -70°C) and once in the body, they can be easily broken down by the immune system before they reach their target.
If you're interested, here's a video by Moderna on how mRNA technology works.
Moderna's mRNA-1273 and Pfizer's BNT162b2 are mRNA vaccines.
Singapore's Covid-19 candidate, ARCT-021, is also an mRNA vaccine. It is developed by Arcturus Therapeutics in collaboration with scientists from the Duke-NUS Medical School.
Both were created based on the genetic sequence of SARS-CoV-2, which the Chinese government released in January 2020.
Because mRNA is easily degraded, Pfizer wraps its BNT162b2 mRNA vaccine in oily bubbles made of lipid nanoparticles so that the vaccine can reach its target in the body.
Have the vaccines been effective?
Data from trials have shown that some of the vaccines highlighted above are somewhat effective, though some results are more (or less) conclusive and convincing than others.
Pfizer and Moderna have reported efficacy rates of between 90 and 95 per cent from their Phase 3 trials.
According to Singapore's Health Sciences Authority, Pfizer's vaccine trial demonstrated a high vaccine efficacy of 95 per cent. This means that there was a 95 per cent reduction of symptomatic Covid-19 disease in a vaccinated group, compared to a non-vaccinated group.
AstraZeneca's vaccine efficacy rate was more wide-ranging: 62 per cent and 90 per cent effective, depending on the level of dose given. The biomedical company's study has actually drawn criticism for apparent lack of rigour and transparency.
Sinovac's CoronaVac has a seroconversion rate of 97 per cent, meaning it triggered antibodies in 97 per cent of trial volunteers. There is no info yet on whether it has the ability to protect against Covid-19. That would have to wait for Sinovac's Phase 3 trial to be completed.
Similarly, Novavax'x Phase 3 trial is not yet completed because of delays related to logistical challenges with scaling up production for said trial.
No vaccine is 100 per cent effective. These efficacy numbers for Moderna, AstraZeneca, and Pfizer are relatively high if we compare them to other vaccines like the ones for influenza (between 40 and 60 per cent) and meningococcal disease (85 to 90 per cent).
Are the vaccines safe to use?
Vaccines typically take eight to 10 years to go through development and rigorous testing before they reach the market.
The entire development and testing process for Covid-19 vaccines has been compressed to under a year because we are now dealing with a global health emergency.
The compressed timeline begs the question:
"Are there side effects from the use of Covid-19 vaccines?"
The most common side effects from vaccines are injection site reactions, fatigue, muscle pain, chills, joint pain, and fever — you would probably experience them if you get the annual flu jab.
These side effects are reportedly more intense in Covid-19 vaccines but they're typically transient.
These adverse reactions were found in Pfizer's Phase 3 trial. Further, four of the participants in the vaccine group reported Bell's Palsy, a condition which causes weakness in facial muscles, after they received the vaccine. However, there is no evidence to prove that it was caused by the vaccine.
A volunteer in the Pfizer trial also reported that he had such severe chills that he cracked a tooth.
On the very day that Pfizer's BNT162b2 was rolled out in the UK, two National Health Service (NHS) workers reported anaphylactoid reactions (where the body's immune system overreacts dangerously) associated with getting the shot. Both workers had a history of allergic reactions.
In response, the UK's Medicines and Healthcare Products Regulatory Agency has warned that the vaccine should not be given to people with a history of anaphylaxis to medicine or food, though Pfizer said that people with a history of severe adverse allergic reactions were already excluded from their late stage trials.
A volunteer in Moderna's Phase 1 testing reported a fever that shot up to 39.4°C after he received the second dose of the vaccine's highest level of dosage. Moderna stopped testing the highest dose after that.
Another trial volunteer in Moderna's study reported that his arm swelled to the size of a goose egg after he received the vaccine.
Again, most of the reported side effects are temporary and are likely an indication that the immune system is doing its job. Hence, most still think that the benefit of being vaccinated against Covid-19 still outweighs the possible side effects.
Will the vaccines work for everyone?
There is not much info on these vaccines' effect on vulnerable sub-groups like pregnant women, and young children, and those with compromised immune systems (for instance, those with HIV).
Case in point: Singapore's Expert Committee on Covid-19 Vaccination cautioned that pregnant women, immuno-compromised persons and those under the age of 16 should not receive the Pfizer-BioNTech vaccine yet, until more data is available.
It is also impossible to tell whether these vaccines will have long-term effects, since none of them are over a year old.
But for many countries now, the potential benefits outweigh the possible risks because there is an urgent need for mass vaccination.
Related story:
We deliver more stories to you on LinkedIn
Mothership Explains is a series where we dig deep into the important, interesting, and confusing going-ons in our world and try to, well, explain them.
This series aims to provide in-depth, easy-to-understand explanations to keep our readers up to date on not just what is going on in the world, but also the "why's".
Top image credit: Daniel Schludi on Unsplash.
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.