Veredus Laboratories, a Singapore biotech firm, has launched two new Covid-19 diagnostic test kits that directly use samples from human saliva or a swab.
How Covid-19 test works now
Speaking in a virtual media conference on Sep. 17, Rosemary Tan, CEO of Veredus, explained that the current prevalent Covid-19 testing relies on nasal swab tests.
When the sample reaches the testing lab, a ribonucleic acid (RNA) extraction phase will occur.
After the RNA sample is extracted, it will then be transcribed and multiplied through a process known as Polymerase Chain Reaction (PCR).
The process of PCR helps to amplify the signals of the virus (if any) so that it becomes more detectable.
The new tests
Saliva Test
The first new Covid-19 diagnostic test that was launched is called the "ZeroPrepTM Saliva Collection Kit".
 Image courtesy of Veredus Laboratories.
Image courtesy of Veredus Laboratories.
When used in conjunction with the PCR kit, is intended for direct testing on human saliva for Covid-19 diagnosis.
Veredus stated in a press release that the kit is able to use human saliva as a specimen for Covid-19 diagnosis as it has a "specially formulated" preservation buffer to stabilise and preserve the viral RNA.
This enables it to be used directly in the PCR test without the need for an RNA extraction, thus saving time.
Veredus added that this specimen collection method is non-invasive as saliva can be obtained easily without assistance as compared to nasal swabs where trained medical personnel are required to perform the procedure.
Hence, this also helps to reduce manpower and eliminate more exposure to the virus.
Swab Test
The second diagnostic test is called the "VereRTTM Covid-19 PCR Kit", which combines the normal PCR kit with a "ZeroPrepTM Swab Buffer Kit".
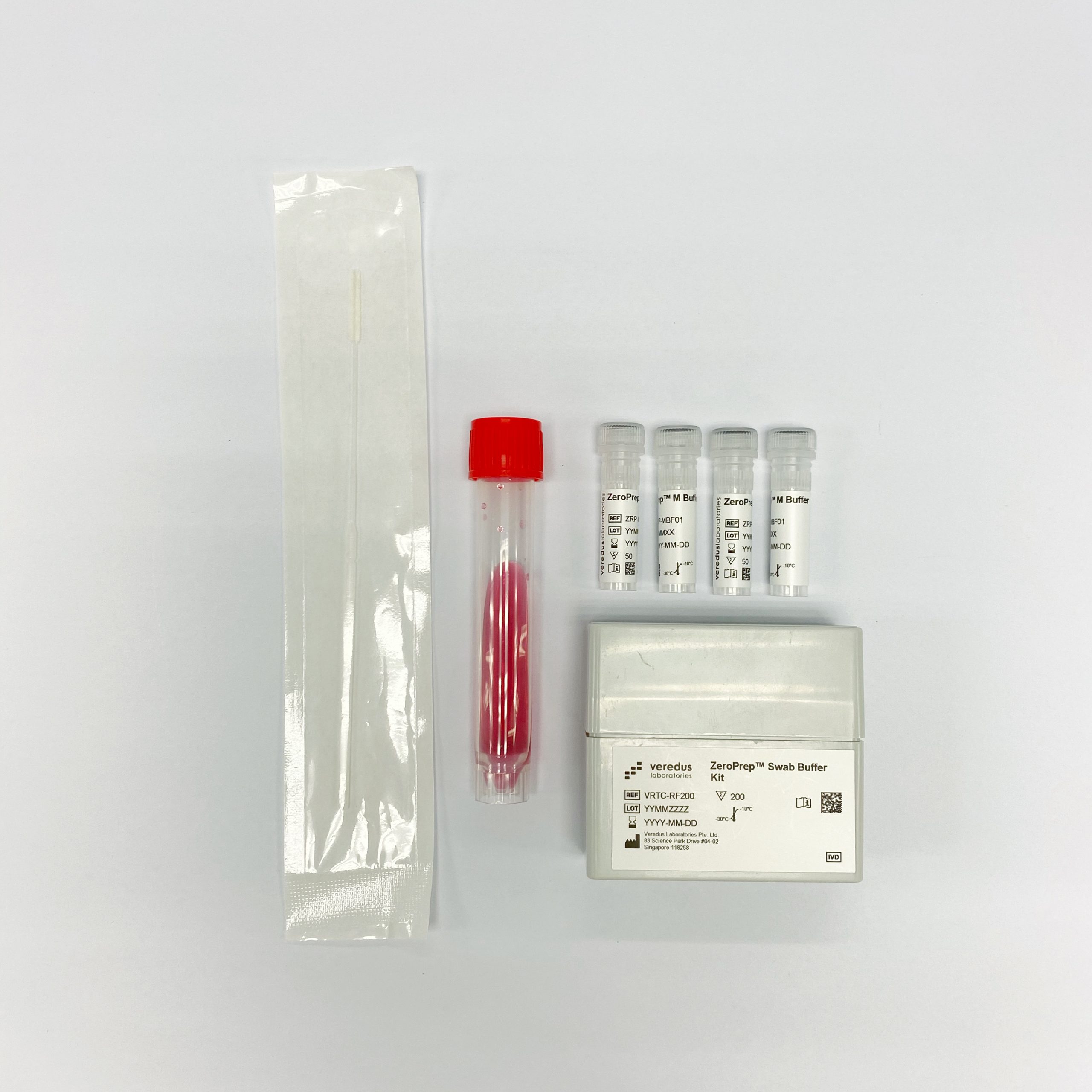 Image courtesy of Veredus Laboratories.
Image courtesy of Veredus Laboratories.
Veredus said that the combination of the two kits is a "next generation" PCR-based In Vitro Diagnostic (IVD) test for Covid-19.
Essentially, it shortens the testing workflow by eliminating the RNA extraction process.
Like the saliva collection kit, this test can also be performed directly from the swab.
By eliminating the RNA extraction step, it saves time and cost, said Veredus.
This also reduces the risk of supply chain shortages, especially during high-volume testing by laboratories globally.
Getting approval from HSA
According to Tan, the ZeroPrepTM Saliva Collection Kit is currently pending approval from Singapore's Health Sciences Authority (HSA).
However, the VereRTTM Covid-19 PCR Kit has obtained a provisional authorisation from HSA.
It can be supplied to hospitals and medical clinics to test patients in Singapore for Covid-19.
Tan said:
"Veredus is innovating rapidly and we are excited to be one of few companies offering direct PCR testing using saliva or swab without the need for viral RNA extraction.
This method of direct Covid-19 testing is the next step for us as we seek to increase the testing capacity of healthcare authorities and laboratories by reducing workflow complexity in Covid-19 molecular testing."
Related stories
Totally unrelated but follow and listen to our podcast here:
Top images courtesy of Veredus Laboratories.
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.
