Scientists at the Nanyang Technological University (NTU) have devised an efficient way to reduce plastic waste.
In a press release, it was revealed that the method could be used to convert non-biodegradable plastic waste into valuable chemicals using sunlight.
First method in the world to break down plastics in only six days
The technique, devised by NTU Assistant Professor Soo Han Sen and his team over two years, uses a catalyst to dissolve plastics in a solvent.
This impressive feat can be achieved in only six days, a remarkably short time for a non-biodegradable material that is known to remain in the environment for hundreds of years.
Plastics are typically hard to break down even with the application of high temperatures, due to their strong and inert chemical bonds.
With this new and more efficient method, Soo and his team have potentially provided the solution to reducing marine plastic pollution.
The amount of plastic sent for incineration in Singapore could be decreased as well, thus extending the lifespan of the Pulau Semakau landfill.
This technique is the first of its kind worldwide.
Although other researchers have developed ways to break down PET plastic (the type of plastic used in plastic bottles) using sunlight, they often require the use of a toxic heavy metal as a catalyst.
The plastics also require longer than six days to break down.
In comparison, NTU's technique takes six days only, and uses an affordable and non-toxic vanadium-based catalyst.
However, it applies only to non-PET plastic, examples of which include styrofoam and most single-use plastic bags.
Chemical has wide range of uses
This method Soo and his team developed uses a clean and limitless source of energy—sunlight—thus helping to reduce the carbon footprint of the technology as well.
It is also a more sustainable alternative to burning plastics.
The method transforms plastic into formic acid, which is commonly used in food preservatives and cleaning products.
 A mixture of the catalyst with dissolved plastics on a plate of plastic granules. Photo courtesy of Lester Kok, NTU
A mixture of the catalyst with dissolved plastics on a plate of plastic granules. Photo courtesy of Lester Kok, NTU
Formic acid can also be used to generate energy in power plants, according to the press release.
When asked if the energy harnessed from formic acid could power plants in Singapore, Soo told Mothership that burning natural gas is a pretty established source of energy, and it was unlikely to be replaced with formic acid anytime soon.
However, he said there are "more viable markets" for formic acid, such as using it directly in hydrogen fuel cells.
These fuel cells can then be utilised in environmentally-friendly electric vehicles such as buses and trains.
Future plans
Currently, the method sees a 100 per cent conversion rate for non-PET plastics.
Moving forward, Soo said the team is working towards applying for research grants to attract talent and scale up their work, as the method is still at least five years away from being commercially viable.
Next steps include looking at how the same technology can be applied to more types of plastics, as well as automating and speeding up the process.
Following these, the scientists will be testing the technology further at Pulau Semakau's Renewable Energy Integrator Demonstrator - Singapore (REIDs) where other forms of renewable energy can be used to power or speed up the process.
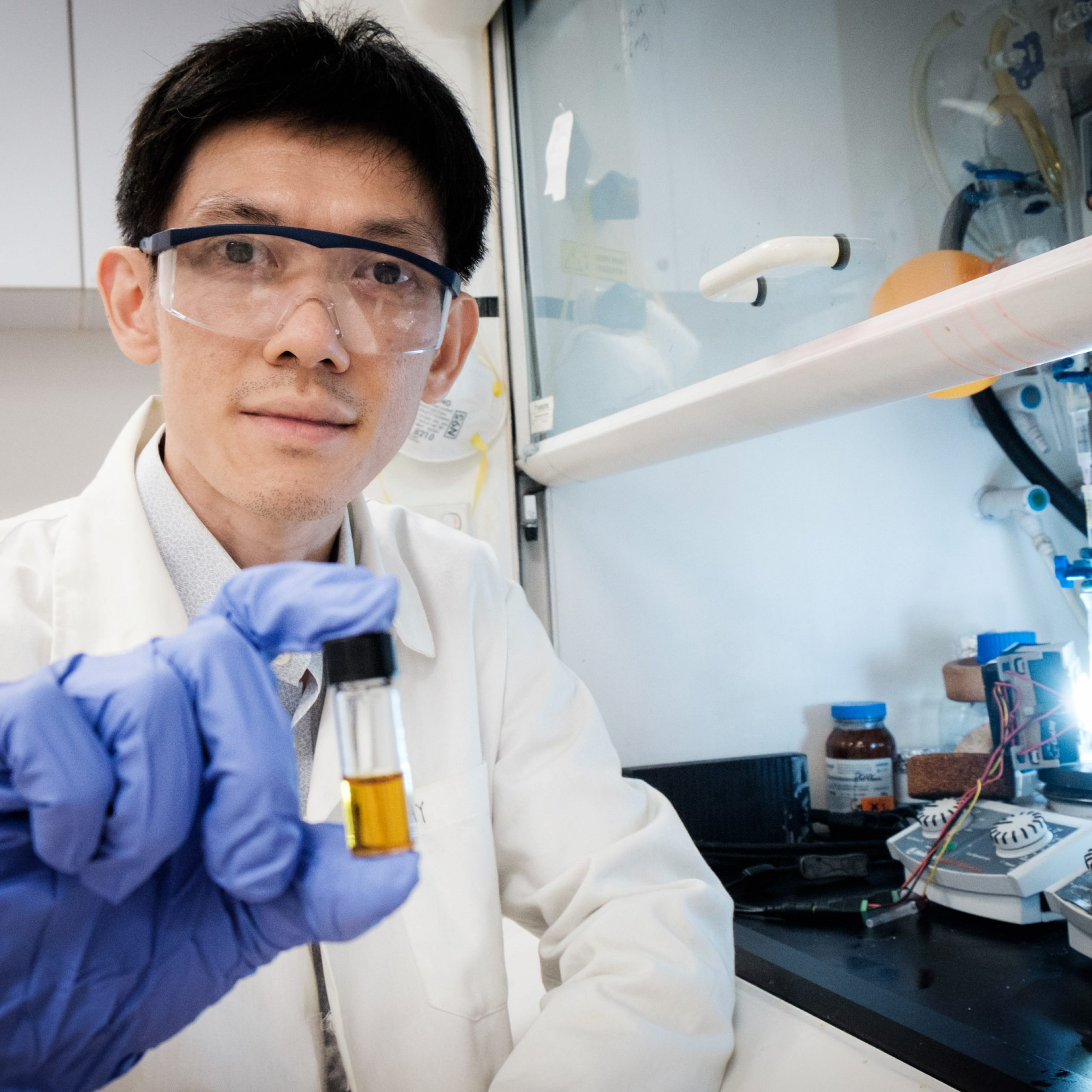 NTU SPMS Asst Prof Soo Han Sen with the plastic-photocatalyst mixture. Photo courtesy of Lester Kok, NTU.
NTU SPMS Asst Prof Soo Han Sen with the plastic-photocatalyst mixture. Photo courtesy of Lester Kok, NTU.
Top photo courtesy of NTU and Pixabay
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.
