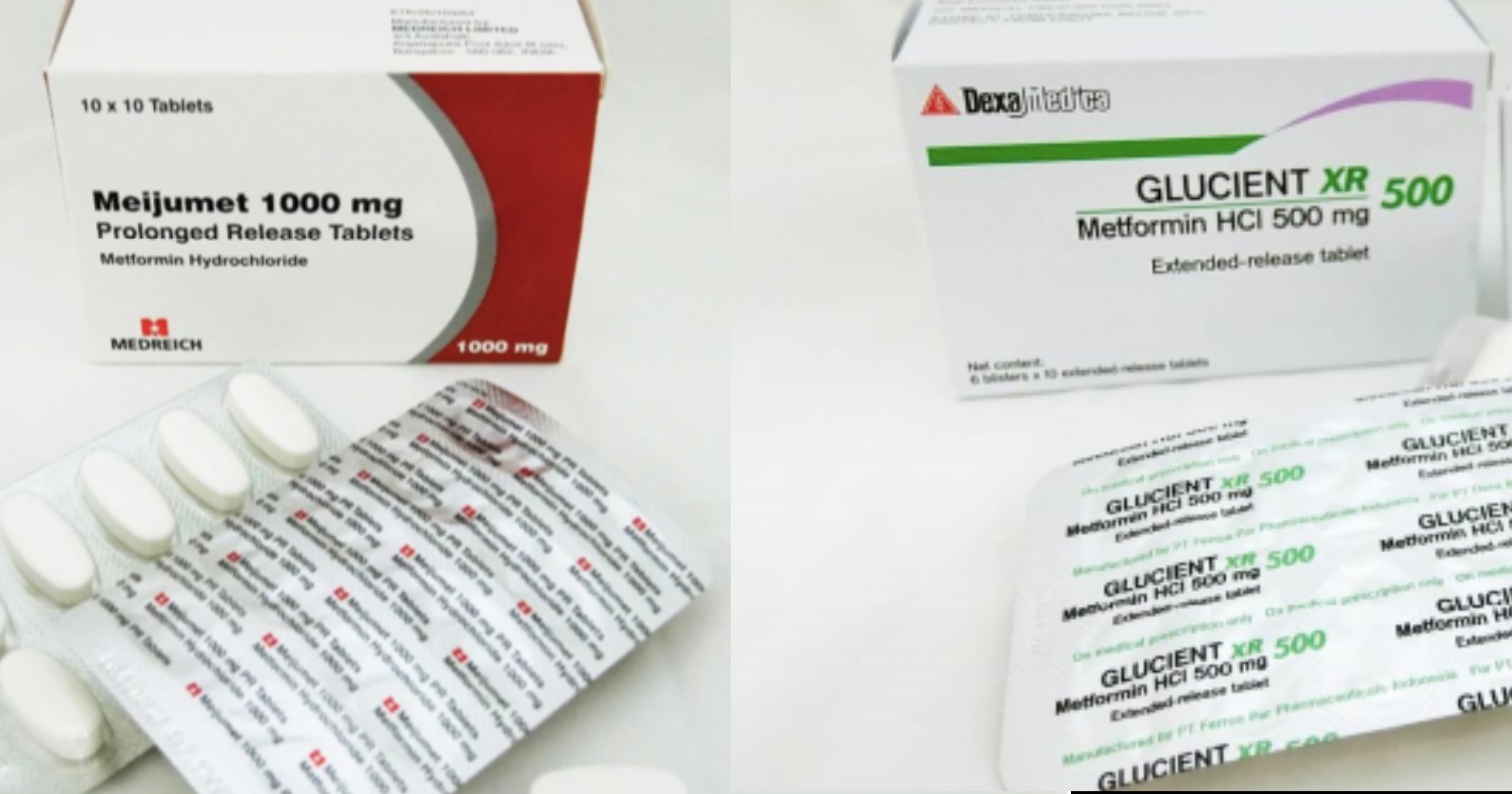
The Health Sciences Authority (HSA) recalled three out of 46 Metformin medicines, after they have been found to contain trace amounts of a potentially cancer-causing impurity.
The recalled medicines are Glucient XR Tablet (500mg), Meijumet Prolonged Release Tablet (750mg) and Meijumet Prolonged Release Tablet (1000mg).
According to a HSA press release on Dec. 4, the other 43 locally-marketed metformin medicines were found to be unaffected after testing was conducted.
Risk to patients quite low
The three metformin medicines were found to contain trace amounts of an impurity known as N-nitrosodimethylamine (NDMA), above the internationally acceptable level.
However, HSA clarified that the risk to patients who have been taking these three affected medicines is very low.
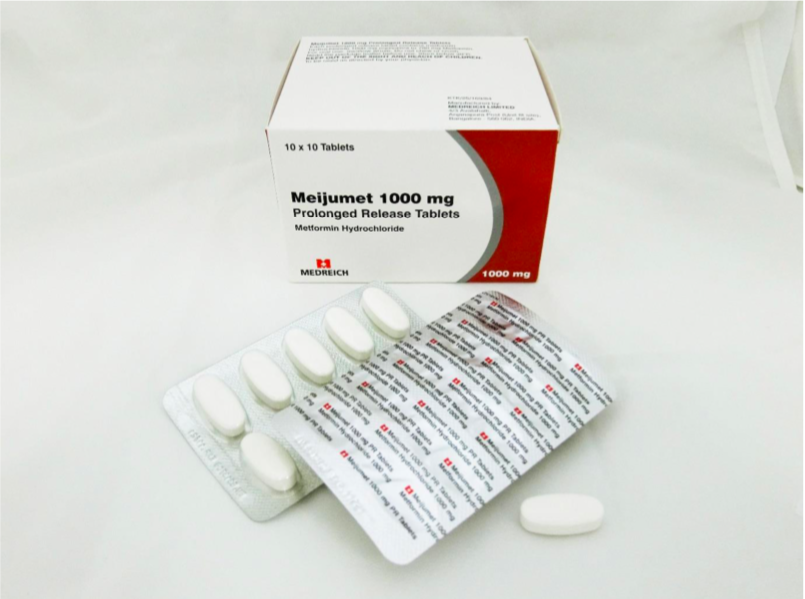 Image from HSA.
Image from HSA.
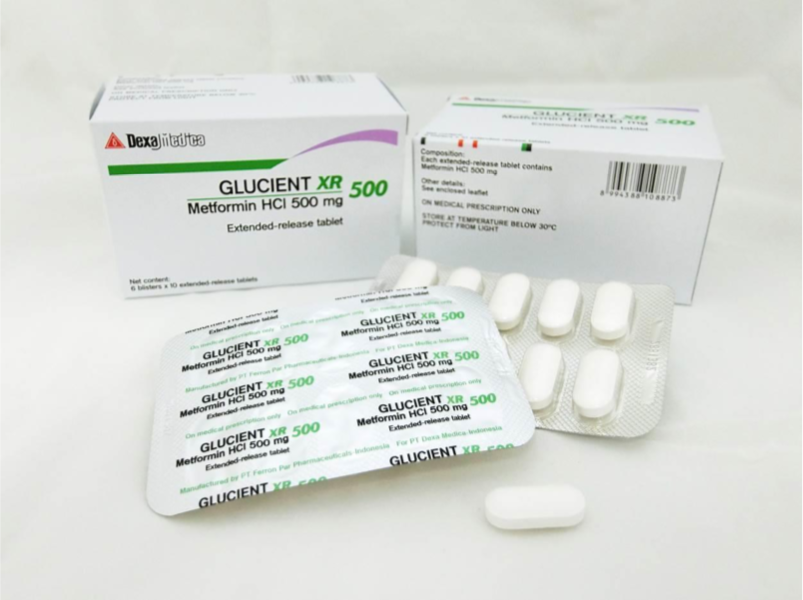 Image from HSA.
Image from HSA.
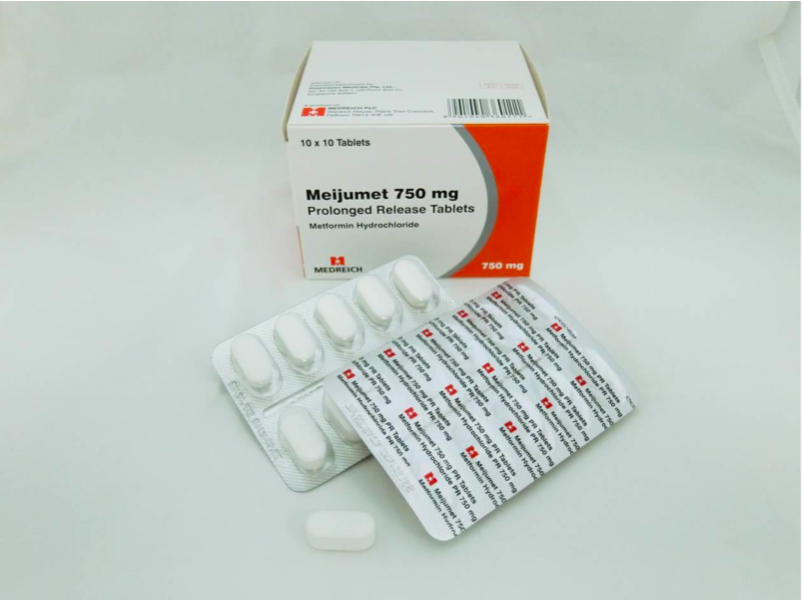 Image from HSA.
Image from HSA.
"This is because the potential risk of nitrosamines is associated with long-term use, and the three affected medicines have only been supplied locally for a short period of time since last year," said HSA.
HSA stated that the added cancer risk from an additional six-month exposure is estimated to be less than 0.00002 per cent.
Patients should not stop treatment on their own
Patients that are currently taking the affected metformin medicines are also advised not to stop treatment on their own, as the sudden stopping of medicines will raise their blood sugar levels.
This may pose a greater health risk to the patient than the trace amounts of NDMA in the affected medicines.
HSA also advised healthcare professionals to contact their patients taking affected medicines as soon as possible, to arrange for an exchange of their medicines.
Patients who are concerned about their current treatment are also advised to consult their doctor and pharmacist for more information.
"HSA is working with the companies supplying these medicines and international regulatory agencies to verify the causes of the contamination, and to identify the necessary measures to address the issue," said HSA.
What is NDMA?
HSA said that NDMA is a type of nitrosamine impurity that can be found in food or the environment, and can be found in low levels in certain kinds of processed food.
They have also recently been found to be formed during the manufacture of some medicines.
According to the U.S. Food and Drug Administration (FDA), NDMA is classified as a probable human carcinogen, which is a substance that could cause cancer.
Recalls had been undertaken worldwide for the affected medicines found to contain these impurities above the acceptable levels.
In September, BBC reported that U.S. based pharmacy CVS suspended a popular heartburn drug Zanatac, over similar concerns over NDMA.
Top image from HSA.
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.