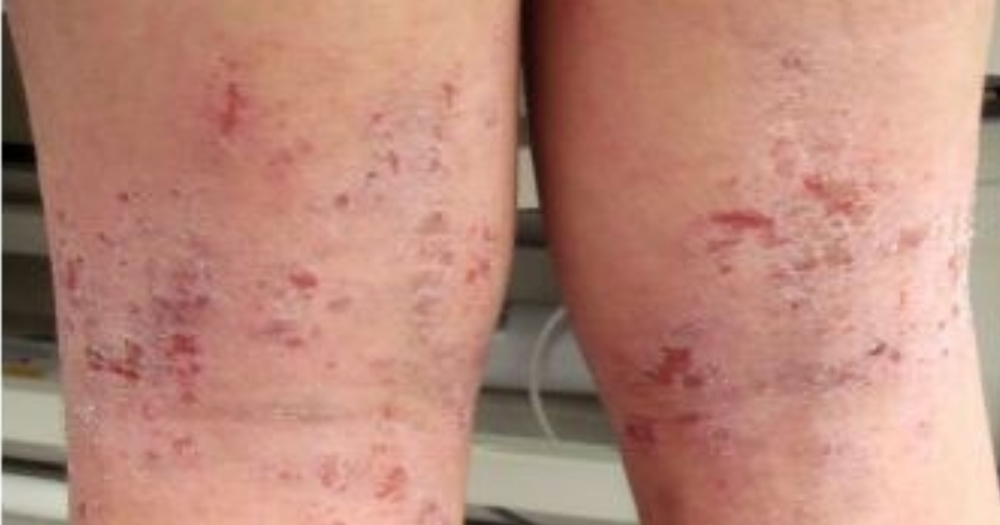
Biopharmaceutical company, Aslan Pharmaceuticals, has made progress in their study on a novel therapeutic antibody that could potentially provide complete relief from eczema symptoms in the future.
New drug can help provide relief to eczema symptoms
The drug known as Aslan004 can inhibit proteins that are key to triggering eczema symptoms such as redness and itchiness.
Currently, available treatments for eczema mostly involve steroid creams or taking antihistamines. Which involves a lot of side effects.
Aslan004 however, according to The Straits Times, is a biologic.
Earlier in June 2019, Aslan completed the first part of the study where the dosage of drug increases for every new cohort.
The results showed that the drug was well tolerated at all doses.
There were no adverse events or discontinuations of the drug during the period.
Analysis of results also showed there was potential for the drug to be administered once every month.
Drug test on moderate to severe eczema patients
Aslan announced on Oct. 22 that they will be doing the second part of the drug test on eczema patients in a randomised manner.
The drug test will also withhold any information that may influence the patients until the end of the test.
The test will also be placebo-controlled that means some people may receive sham "placebo" that poses no real effect on the skin conditions.
The test will be conducted at Singapore's National Skin Centre and Changi General Hospital, led by Prof Steven Thng.
Each participant will receive multiple doses of the drug, this is known as a multiple ascending dose (MAD) study.
The MAD study will evaluate three doses of the drug, delivered subcutaneously, followed by an expansion of the cohort at the most effective dose.
The study will recruit up to 50 moderate to severe eczema patients.
According to ST, Thng noted that it was "highly unlikely" for a cure to eczema anytime soon, Aslan004 would make treatment for patients more convenient, especially since there are limited treatments for those with severe eczema.
Results are expected in the second half of 2020.
Top photo from Singhealth website
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.