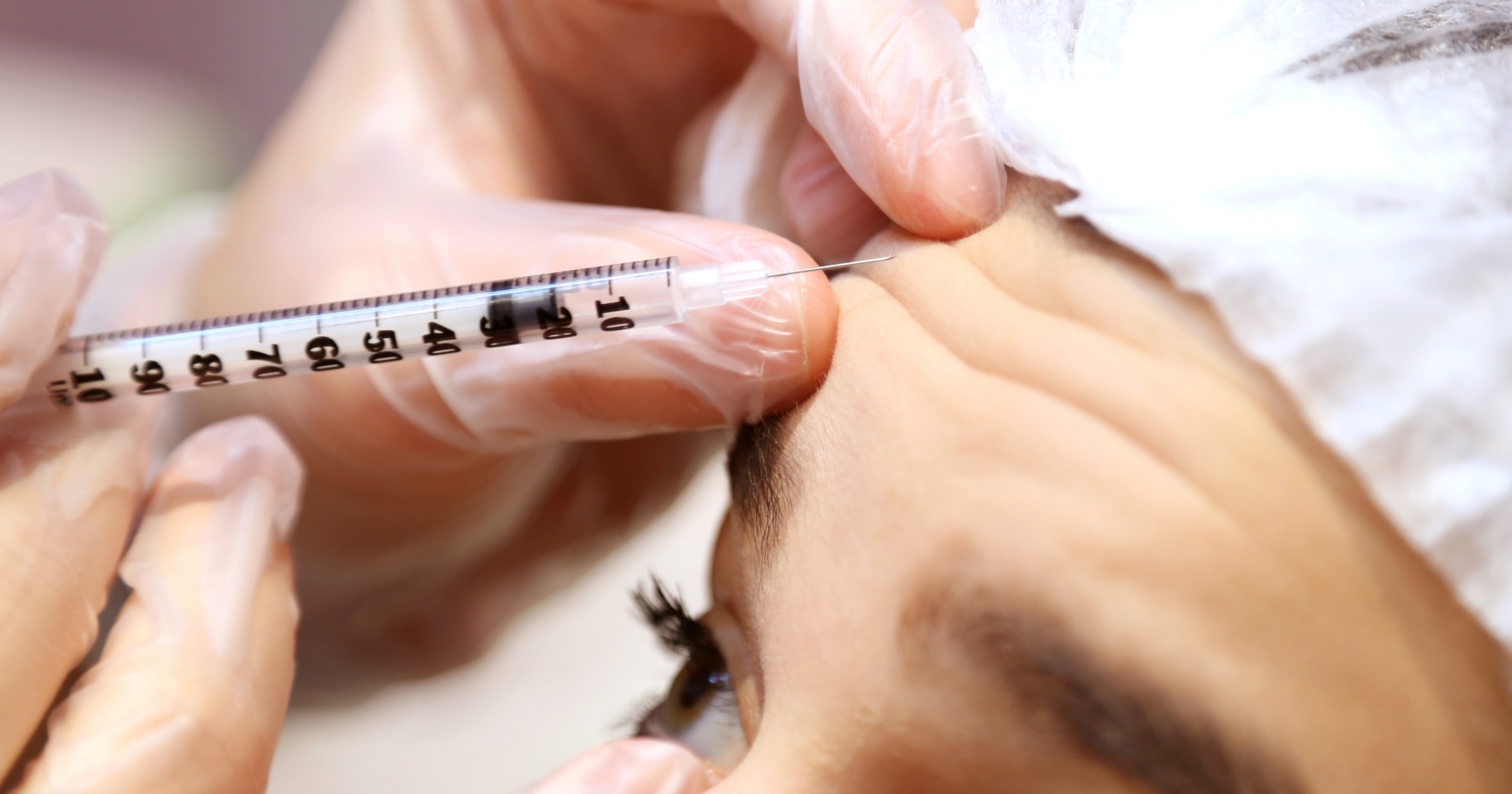
A woman in Singapore became blind after receiving AestheFill, a brand of dermal filler.
The Health Sciences Authority (HSA) was notified about the incident and it is currently investigating the matter.
What happened?
The incident happened on Jul. 29, 2023.
The woman, reported to be young and married, received the dermal filler injection at a clinic in Redhill.
'The Straits Times reported that the doctor who performed the injection is a certified trainer in AestheFill injections.
AestheFill is used to temporarily improve facial wrinkles and folds.
It is injected directly into the subcutaneous layer of facial skin
After receiving the injection, the woman reportedly went blind in both eyes.
Parvus, the company that distributes AestheFill in Singapore, reported to HSA an incident of blood vessel occlusion, which means blockage of blood vessels.
Known risk for dermal fillers
An HSA spokesperson told Mothership that blood vessel occlusion is a known risk for dermal fillers.
Blood vessel occlusion resulting in blindness is commonly listed as a potential adverse event in the Instructions For Use, which are provided to clinicians.
Specifically, AestheFill's Instructions For Use caution against injecting the product into blood vessels as it may cause a blockage.
The spokesperson also listed other common complications that have been reported with the use of dermal fillers: swelling, redness, raised bumps in or under the skin (nodules or granulomas), skin blanching (associated with injection into blood vessel), as well as temporary blurring of vision.
Dermal fillers are classified by the HSA as Class D medical devices, which is the highest risk class.
Clinicians who administer dermal fillers are required to undergo training.
"Consumers are advised to discuss with their clinician about the risks and suitability of the dermal fillers before going for the procedure," said the spokesperson.
HSA investigating for batch-related defects
HSA requires companies that supply medical devices to report adverse events within 10 days.
This is the first adverse event report for blindness resulting from the use of dermal fillers locally.
AestheFill has been registered in Singapore since Oct. 1, 2021.
HSA is currently investigating if there are any batch-related defects that may have affected the product safety or quality.
"Should there be any product or batch-related issues, HSA will take the necessary actions such as to recall the affected product and/or require the company to rectify the issues," said the spokesperson.
Doctor reportedly uncontactable
ST reported that its attempts to contact the doctor who administered the injection were unsuccessful.
A general manager from Parvus told the paper that it is in contact with all parties involved and "working diligently to understand the circumstances" of the incident.
Mothership has reached out to Parvus to ask about the steps it has taken to assist the affected customer.
Top photo: Vlad Egorov on Unsplash
If you like what you read, follow us on Facebook, Instagram, Twitter and Telegram to get the latest updates.